Have you ever wondered why ice is slippery when you skate? Probably not, but now that the question is out there could you answer it? Scientists of the past rationalized that the pressure on the ice (applied for instance by an ice skater) caused the top layer of the ice to melt, in theory leading to a small layer of water to form on the ice and voila the ice becomes slippery. If you are wondering why this is a convincing argument well, the answer is that water is less dense as a solid (ice) than a liquid which means it holds the ability to have a lowered melting point caused by pressure. BUT, what if the answer is not that simple? In today's scientific community this simple explanation is not widely accepted. The point is that the pressure of the skates will only lower the melting point of the ice by a very, very small percentage. With that being said the answer is sort of unknown, some scientists think that the friction from the skates melts the ice (but this leaves the door open to questions like what if the person is NOT moving at all is the friction still a valid answer?), while other scientists think that the water on top of the ice is a natural occurrence made of unstable water molecules searching for stability (meaning they are in constant random motion).
ANSWER #1: Unknown! (However, the theories of a thin watery layer on top of the ice or friction and even some pressure probably play into the slipperiness of ice)
Websites Consulted;
http://www.livescience.com/32507-why-is-ice-slippery.html
http://dujs.dartmouth.edu/questions/what-causes-ice-to-be-slippery#.VoGSOpMrJEI
QUESTION #2: Is foam a liquid, solid or gas?
To really understand foam try holding it, can you make the determination -- is foam a solid, gas or a liquid (use the chart above if you want to review the basic properties of solids, liquids and gases).We use it every day when washing dishes, shaving, taking a bubble bath but do we really know the weird foam that permeates our lives? The answer is no. Actually as far as research has shown me no real theories exist on the stiffness of foam when looking at variables like bubble sizes or ingredients. Ironically, this is valuable information when for instance filling the cracks in buildings (no one wants bugs to easily squeeze through into their home). So, we know it is important but do we know it on the most basic of levels, can we determine if it is in fact a solid, gas or a liquid. There are many people who think foam is either a liquid or solid one or the other simply aerated with a gas. But this is because they are looking at the components, not the characteristics. When looking at a simple shaving cream well the line is harder to draw, since it holds it shape like a solid for a short period of time however, when left overnight it losses its shape being left as a layer of soap. But, unlike solids it will take the shape of a container (shaving cream containers being a prime example). This leads to the question, if a aerated liquid shows properties and characteristics of both liquids and solids what exactly is it?
*Note, in this example we were looking at liquid aerated foams NOT solid aerated foams like sponges which are actually solids with many air holes.
ANSWER #2: Liquid Aerated foam has the properties of both solids and liquids, but has a bunch of gas in it, so it is hard to say, it can be argued depending who you are and how you classify a compound.
Websites Consulted:
https://www.quora.com/Is-foam-a-liquid-a-solid-or-a-gas
http://mypages.iit.edu/~smart/hallden/lesson1.htm
https://en.wikipedia.org/wiki/Foam
QUESTION #3: Why are cheerios magnetic?
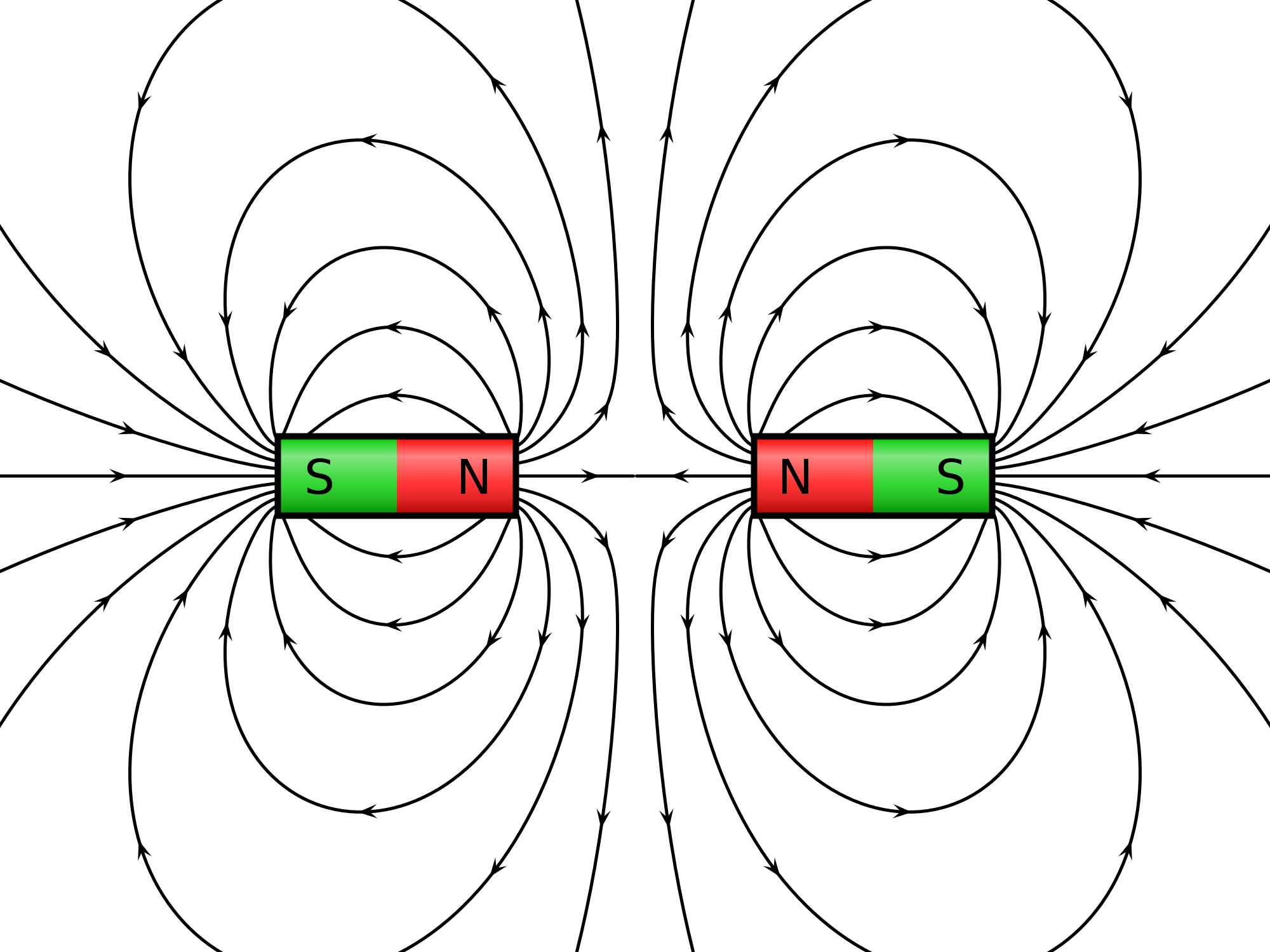
Alright, so we've answered one of the magnetic questions about cheerios but not the other, why can I use a magnet and pull a cheerio through water? There are two answers, one being that breakfast cereal is fortified with iron and iron is attracted to magnets. If you crush the cereal into a fine dust and put a magnet to it then you will see some of the cereal bits sticking to the magnet therefore it isn't the water but the ingredients of the cereal leading to the magnetism. However, other objects with NO iron content will also be 'attracted' towards a magnet when left to float on water, the reason is the diamagnetic property of water meaning the water when exposed to a magnetic will actually produce a magnetic field in the opposite direction as the one produced by the magnet in essence the water is repelled by the magnet leading to an dent being made for the non-magnetic object to fall into, the object will continue to fall into the indent as the magnet is drawn across the water surface.
What the two magnetic field of water vs. magnet create, diamagnetism is why there is an indent in the water.
ANSWER #3: Diamagnetism and IRON
Websites Consulted:
https://en.wikipedia.org/wiki/Cheerios_effect
http://www.livescience.com/9350-cereal-science-floating-objects-stick.html
http://www.damtp.cam.ac.uk/user/dv211/cheerios.html
http://io9.gizmodo.com/5514825/the-cheerio-effect
https://www.kjmagnetics.com/blog.asp?p=cereal-contains-iron